What is electricity?
Electricity plays a big part in our lives, it lights our homes, we cook our food, it powers our telephones and computers. To understand how electricity works we need to understand how Atoms work.
Atoms
To answer what is electricity we need to understand Atoms. Everything on Earth is made up of so-called atoms. We cannot see the atoms with our own eyes without a powerful microscope. The atoms are made up of three small parts. These three are called protons, neutrons, and electrons. Atoms have a nucleus, the center of the atom where the protons and neutrons are found. The neutrons have no charge, and protons have a positive charge. The electrons which orbit around the nucleus have a negative charge, these electrons are found in areas called shells. A shell is sometimes called an energy level.
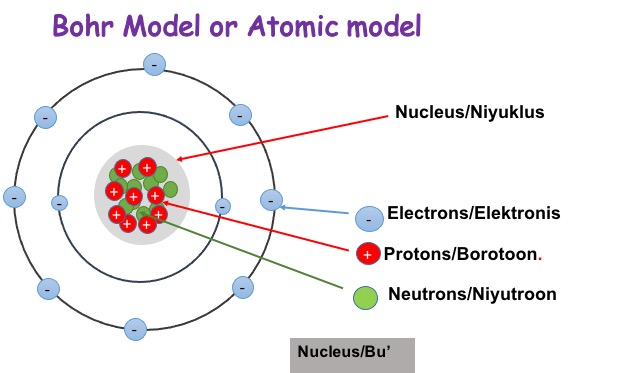
Every atom has at least one proton in it is the nucleus. The protons are very important because we can differentiate one atom from another atom by the number of protons they have in the nucleus. The number of protons is called Atomic Number. For example copper has 29 protons, hydrogen has one proton.
Electron Shells
An electron shell, or energy level, is the part of an atom where electrons are found orbiting the atom’s nucleus. Atoms have one or more electrons in the shells.
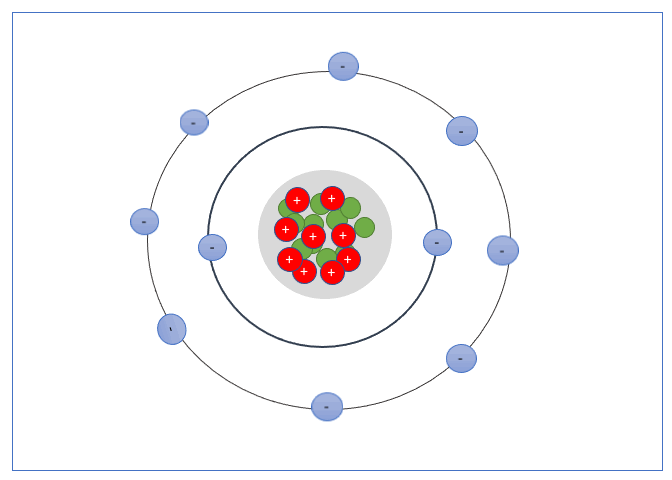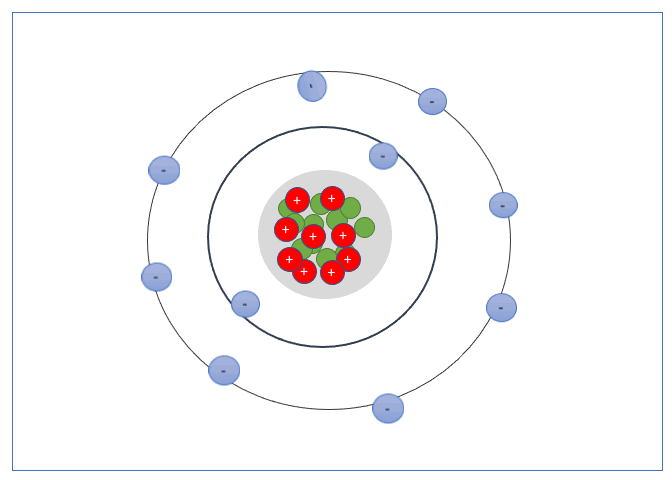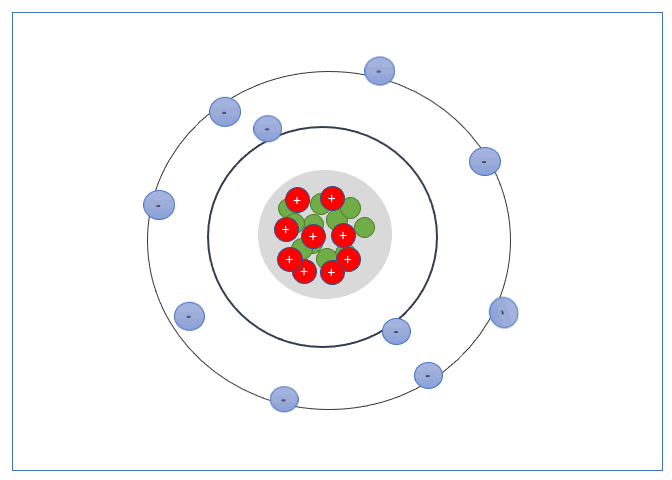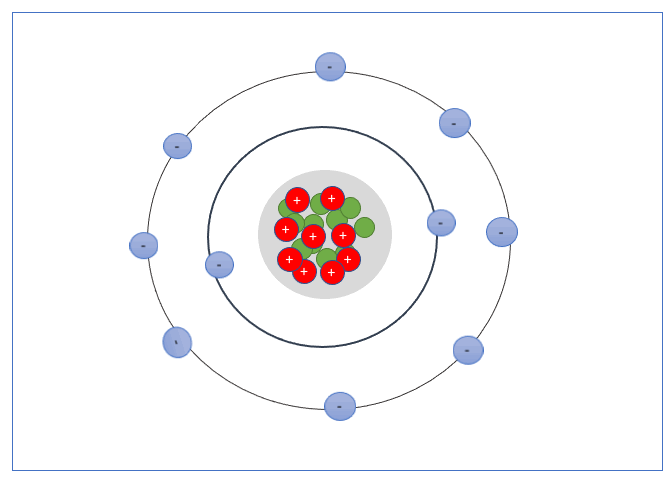
These Shells can only take a specific number of electrons. The first shell can hold 2 electrons, the second shell 8 electrons, the third shell can hold up to 18, and fourth can hold up to 32, and so forth.

The valence shell is the outermost shell of an atom, the electrons found in the valance shell are called valance electrons, and the number of electrons it contains strongly influences the electrical properties of that element or material.
Conductors
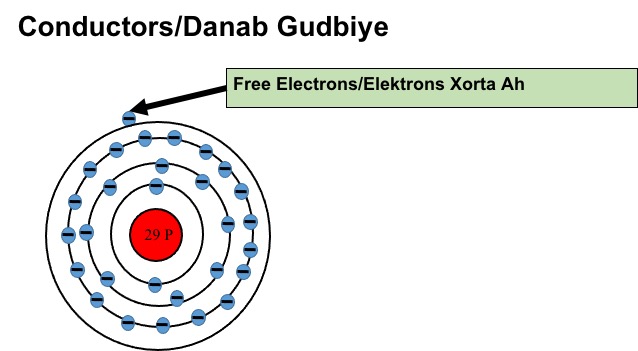
Some materials let electricity pass through them easily. These materials are known as electrical conductors, they have loosely bound electrons in their valance shell which can move from one atom to another. These moving electrons are called free electrons and are specific to Conductors. All metallic structures have free electrons.

Copper is a good example of a good conductor. Copper has 29 electrons, the first shell can hold 2 electrons, the second shell 8 electrons, the third shell can hold up to 18, and the fourth will hold only 1 electron in its valance shell. This totals 28 electrons with 1 electron left in the valance shell. The valence shell ideally needs 8 electrons to be full but copper has only one. Therefore copper has 1 free electron to give. Most of the electrical wires and components have copper within them. The best conductors are Silver, Copper & Gold. All have one valence electron in their valence shell.
Electric current (Electricity)
An electric current is the flow of these free electrons in one direction from a negative terminal to the positive terminal. An energy source for example a battery cell is required to make the free electrons travel in one direction.

The direction of current flow
Electron current and conventional current are two types of notation we use for current flow in a circuit.
Electron flow, electrons flow from negative terminal to positive terminal. Conventional current flow is the opposite of electrons flow, from positive to negative terminal.

In this tutorial, I have just introduced basic knowledge about electricity and answered few questions. To further increase your knowledge about electricity, please watch the Electricity Video.